June 22, 2023
Reading time • 5 min
Anvisa warns of medicine counterfeiting cases
Anvisa determined, on this Wednesday (21/6), the seizure and prohibition of the commercialisation, distribution and use of counterfeit units of the medicines Botox®️ (Lot: C3709C3) and Durateston® (Lot: 701012LR). The measure was published through Resolution RE 2,198/2023.
This Thursday (22/6), through Resolution-RE 2.208/2023, the seizure and prohibition of the commercialisation, distribution and use of counterfeit units of batch W07209 of the product Dysport®️ were also determined.
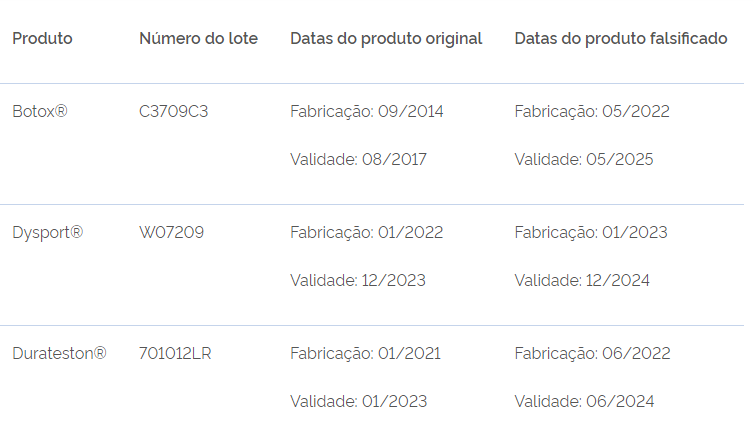
The products can be differentiated by the manufacturing and expiry dates, which are different from the original products. See a summary of the differences in the table and learn more details below.
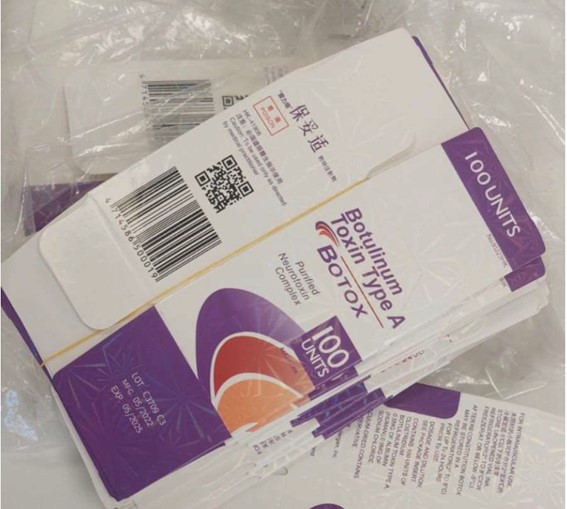
Counterfeit Botox – batch C3709C3
The measures were taken after a police operation that found counterfeit Botox®️ packaging.
The manufacturing date of the original batch (C3709C3) is 09/2014 and the expiry date 08/2017. The counterfeit product has the same batch number, but its manufacturing date is 05/2022 and the expiry date is 05/2025, as shown below.
In addition, the original batch (C3709C3) was not intended for the Brazilian market and is therefore a counterfeit. The medicine Botox®️ is duly registered in the name of the company Allergan Produtos Farmacêuticos.
Counterfeit product packaging.
Counterfeit Dysport 300U (botulinum toxin A) – batch W07209
The measures were taken following communication from the company holding the registration for the medicine, Beaufour Ipsen Farmacêutica, to Anvisa.
The company clarifies that batch W07209 of the original product Dysport®️ 300U was imported in April 2022, with a manufacturing date of 01/2022 and an expiry date of 12/2023. The counterfeit product has a manufacturing date of 01/2023 and an expiry date of 12/2024.
The company also described several differences between the original product and the counterfeit. Discrepancies were found in the type of bottle, the quality of the label printing and the volume of product in the bottle, as shown in the photos below.
It is important to clarify that the product Dysport®️ 300 U, batch W07209, manufactured in January 2022 and with an expiry date of December 2023, is genuine and there is no problem related to the quality, efficacy and safety of the product, which may be available on the market.

Counterfeit product: differences in the label
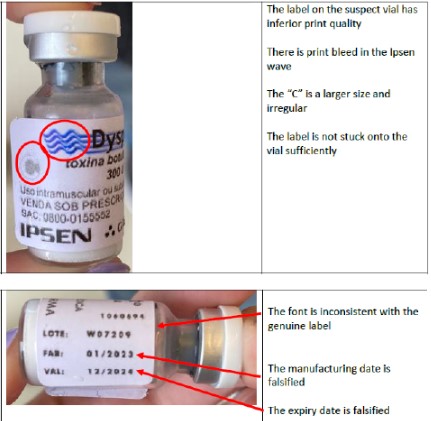
Counterfeit product: differences in the bottle
Durateston® counterfeit – batch 701012LR
The measures were taken after Anvisa was informed by the company Aspen Pharma Indústria Farmacêutica Ltda. about the circulation, in the Brazilian market, of counterfeit units of the medicine.
The number 701012LR corresponds to an original batch number of Durateston®, manufactured on 01/2021 and valid until 01/2023. However, the counterfeit units have divergent manufacturing and expiry dates (manufactured in 06/2022 and valid until 06/2024), as shown in the photo below.

Counterfeit product packaging.
General guidelines for the population and health professionals
Anvisa advises that the population and health professionals only purchase medicines in duly regularised establishments, always in the complete packaging (inside the box) and upon issuance of the invoice.
In case of identification of units of medicines suspected of being counterfeit, the population or health professionals should not use the product and should contact the companies that hold the registration of these products to verify their authenticity.
In addition, the fact must be reported immediately to Anvisa, preferably through the Notivisa system (in the case of health professionals) or through the Ombudsman system, using the FalaBR platform (in the case of patients).
« Home